- Product Details
Keywords
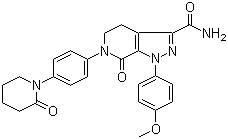
- 1-(4-Methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-yl)phenyl]-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyr
- supply Apixaban
- Apixaban 99.0%
Quick Details
- ProName: Apixaban
- CasNo: 503612-47-3
- Molecular Formula: C25H25N5O4
- Appearance: white powder
- Application: API
- DeliveryTime: delivery after confirming an order
- PackAge: aluminium foil bag; drum; as per your ...
- ProductionCapacity: Metric Ton/Day
- Purity: 99.0%
- Storage: store in cool place. Keep container ti...
- Transportation: air transport, sea transport
- LimitNum: 1 Gram
Superiority
Details
Trading information
| Product Name | Apixaban |
| Synonyms | 1-(4-Methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-yl)phenyl]-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxamide |
| CAS No. | 503612-47-3 |
| Molecular Formula | C25H25N5O4 |
| Molecular Weight | 459.50 |
| Appearance | white powder |
| Purity | 99.0% |
| Package | aluminium foil bag; drum |
| Payment Terms | L/C; D/A; D/P; T/T |
| Delivery | by air; by ship |
.jpg)
Sample Service
Considering the various needs of buyers, we provide small quantity sample to customers. You are welcomed to contact us for the detailed specifications of our APIs and pharmaceutical intermediates. Customers are also expected to confirm the type of freight transport and payment. Any more question please contact us directly.
.jpg)
Medical Uses of Apixaban
Apixaban (INN, trade name Eliquis) is an anticoagulant for the prevention of venous thromboembolism and venous thromboembolic events. It is a direct factor Xa inhibitor. Apixaban has been available in Europe since May 2011 and was approved for preventing venous thromboembolism after elective hip or knee replacement. The FDA approved apixaban in December 2012 with an indication of reducing the risk of stroke and dangerous blood clots (systemic embolism) in patients with atrial fibrillation that is not caused by a heart valve problem. The drug was developed in a joint venture by Pfizer and Bristol-Myers Squibb.
Pharmacodynamics of Apixaban
Apixaban acts by inhibiting coagulation, and thus prevents development of blood clots. As a result of FXa inhibition, apixaban prolongs clotting tests such as prothrombin time (PT), INR, and activated partial thromboplastin time (aPTT). Changes observed in these clotting tests at the expected therapeutic dose, however, are small, subject to a high degree of variability, and not useful in monitoring the anticoagulation effect of apixaban. The Rotachrom Heparin chromogenic assay is not recommended for assessing the anticoagulant effect of apixaban.
Research and Development
Relying on advanced research platform and relative service of China Medicine City, Allyrise have made achievements in areas of antineoplastic, antiviral, diabetes drugs in recent years, with more than 20 kinds of APIs and advanced intermediates magnified in pilotscale experiment. The products are widely sold to famous pharmaceutical companies from around the world. To enhance our research and development level, Allyrise developed close cooperation with foreign pharmaceutical companies in customer synthesis and developing new drugs.
Technical Team
Jiangsu Allyrise Pharmaceutical Co., Ltd have a young and innovative technician team with average age of 29 and backgrounds of famous universerties. We have rich experience in lab research and pilot scale experiment, laying the foundation for further personnel cultivation.
.jpg)




![AcetaMide, N-[(1R)-1-(4-broMophenyl)ethyl]-2,2,2-trifluoro-](http://file1.lookchem.com/300w/substances/2022-02-21-10/1ff12cc5-0d08-4ffa-a802-dc535dc9ab1d.png)
![(αR)-α-[(1R)-1-Aminoethyl]-α-(2,4-difluorophenyl)-1H-1,2,4-triazole-1-ethanol Hydrochloride](http://file1.lookchem.com/cas/2024/03/06/1391468-05-5.jpg)