- Product Details
Keywords
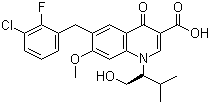
- 6-(3-Chloro-2-fluorobenzyl)-1-[1(S)-(hydroxymethyl)-2-methylpropyl]-7-methoxy-4-oxo-1,4-dihydroquino
- JTK 303
- GS 9137
Quick Details
- ProName: Elvitegravir
- CasNo: 697761-98-1
- Molecular Formula: C23H23ClFNO5
- Appearance: off-white powder
- Application: API
- DeliveryTime: delivery after confirming an order
- PackAge: aluminium foil bag; drum; as per your ...
- ProductionCapacity: Metric Ton/Day
- Purity: 99%
- Storage: store in cool place. Keep container ti...
- Transportation: air transport, sea transport
- LimitNum: 1 Gram
Superiority
Details
Trading information
| Product Name | Elvitegravir |
| CAS No. | 697761-98-1 |
| Molecular Formula | C23H23ClFNO5 |
| Molecular Weight | 447.88 |
| Appearance | off-white powder |
| Purity | 99.0% |
| Package | aluminium foil bag; drum |
| Payment Terms | L/C; D/A; D/P; T/T |
| Delivery | by air; by ship |
.jpg)
Sample Service
Considering the various needs of buyers, we provide small quantity sample to customers. You are welcomed to contact us for the detailed specifications of our APIs and pharmaceutical intermediates. Customers are also expected to confirm the type of freight transport and payment. Any more question please contact us directly.
.jpg)
Usage of Elvitegravir
Elvitegravir (EVG) is a drug used for the treatment of HIV infection. It acts as an integrase inhibitor. It was developed by the pharmaceutical company Gilead Sciences, which licensed EVG from Japan Tobacco in March 2008. The drug gained approval by U.S. Food and Drug Administration on August 27, 2012 for use in adult patients starting HIV treatment for the first time as part of the fixed dose combination known as Stribild.
According to the results of the phase II clinical trial, patients taking once-daily elvitegravir boosted by ritonavir had greater reductions in viral load after 24 weeks compared to individuals randomized to receive a ritonavir-boosted protease inhibitor.
Technical Team
Jiangsu Allyrise Pharmaceutical Co., Ltd have a young and innovative technician team with average age of 29 and backgrounds of famous universerties. We have rich experience in lab research and pilot scale experiment, laying the foundation for further personnel cultivation.
.jpg)
Research and Development
Relying on advanced research platform and relative service of China Medicine City, Allyrise have made achievements in areas of antineoplastic, antiviral, diabetes drugs in recent years, with more than 20 kinds of APIs and advanced intermediates magnified in pilotscale experiment. The products are widely sold to famous pharmaceutical companies from around the world. To enhance our research and development level, Allyrise developed close cooperation with foreign pharmaceutical companies in customer synthesis and developing new drugs.
Escrow Service
Allyrise accept Escrow Service which is based on Lookchem as a third-party. Both buyer and seller are guranteed that their transaction is simple and secure.
.jpg)




![AcetaMide, N-[(1R)-1-(4-broMophenyl)ethyl]-2,2,2-trifluoro-](http://file1.lookchem.com/300w/substances/2022-02-21-10/1ff12cc5-0d08-4ffa-a802-dc535dc9ab1d.png)